1. NJOY sues disposable e-cigarette manufacturers
NJOY has filed lawsuits against 34 manufacturers, distributors and online retailers of illegal disposable e-cigarette products that are illegally marketed and sold in California and other U.S. states. The lawsuit alleges that the companies manufacture, distribute, market and sell products that violate California’s flavor ban law, are illegal under federal law and are subject to sanctions by the U.S. Food and Drug Administration (FDA) and are inconsistent with compliance with state and federal laws. companies engaging in illegal competition.
The lawsuit seeks a nationwide ban on the import, marketing and sale of these illegal products and seeks substantial compensatory and punitive damages.
“These companies knowingly violated federal and state laws and need to be held accountable,” Murray Garnick, executive vice president and general counsel of NJOY parent company Altria Group, said in a statement. “There are two markets today. ——One is a market for those who follow the rules, and the other is a market for those who blatantly ignore the rules. We are taking this action because the current situation in the illegal e-cigarette market is intolerable, and we must see the FDA and other agencies Take more action.”
The lawsuit, filed in the U.S. District Court for the Central District of California, alleges four counts: unfair competition, false advertising, false advertising in violation of the Lanham Act, and violations of the All Cigarette Trafficking Prevention Act of 2009.
The defendants named in the lawsuit manufacture and distribute illegal disposable e-cigarette products, including Breeze, ElfBar, EB, EBCreate, EscoBar, Flum, JuiceBox, LavaPlus, Loon, Lost Mary, MKG,Mr. Fog and Puff bar brands. U.S. defendants include companies doing business in Arizona, California, Delaware, Florida, Michigan, Minnesota, New Jersey, New York and Texas. The foreign defendants all reside in China.
NJOY stated that none of the defendants had obtained premarket authorization from the FDA.
Despite a ban on the sale of flavored tobacco products taking effect in December 2022, flavored e-cigarette products still account for more than 97% of the California market, according to a recent study commissioned by Altria.
NJOY’s action against the disposable vapor product manufacturer follows a complaint filed by RJ Reynolds Tobacco Company with the International Trade Commission. Charge unfair import fees to multiple disposable vaping device manufacturers, distributors and retailers.
2. Altria unit says dozens of e-cigarette manufacturers sell illegal products
Altria subsidiary NJOY asked a California federal court on Thursday to ban more than 30 e-cigarette product manufacturers from selling their products in the United States, claiming they peddled products that violated state and federal laws and illegally competed with companies that followed the rules.
E-cigarette manufacturer NJOY LLC said in the lawsuit that it is seeking a nationwide injunction prohibiting the import, marketing and sale of “illegal products” and demanding compensation for significant damages. The complaint names dozens of domestic and Chinese manufacturers, retailers and distributors of popular disposable e-cigarette brands including ElfBar, EscoBar, PuffBar and JuiceBox.
According to NJOY, these products violate California’s ban on flavored tobacco products, as well as California’s False Advertising Act, the Lanham Act and the All Cigarette Trafficking Prevention Act of 2009. The U.S. FDA has repeatedly taken action against many of these companies, and NJOY said in the lawsuit that they continue to sell their products.
NJOY said this is a problem because these companies do not tell consumers that their products are illegal or subject to FDA sanctions.
“Instead, the defendants represent, explicitly or implicitly, that their [flavored disposable e-cigarette devices comply with regulatory and legal requirements and may be lawfully sold in California and elsewhere,” he said.
The statement said that these statements are false and misleading and that continuing to sell this product is unfair to companies like NJOY that comply with California’s flavor ban and other federal laws.
NJOY said it invested significant time and resources to develop legal products and obtain the necessary approvals. Meanwhile, “significant numbers” of consumers have purchased and continue to purchase illegal products instead of NJOY’s legal products. This resulted in lost sales, lost profits, and other financial losses, he added.
The statement said: “This injury is a direct result of Defendants’ illegal, unfair and deceptive conduct, without which their products would not be sold, and without relief from the court, Plaintiffs will continue to suffer harm.” Altria Group
Murray Garnick, executive vice president and general counsel, said in a statement Thursday that the companies targeted in the lawsuit “need to be held accountable.” “Today, there are two markets – a market for those who follow the rules and a market for those who blatantly ignore them,” Garnick said. “We are taking this action because of the current state of the illegal e-cigarette market. This is intolerable and we must see more action from the FDA and other agencies.”
According to the statement, none of the defendant company’s products has received premarket authorization from the FDA, nor has it submitted the required premarket approval application. NJOY said several of the companies had received warning letters from the FDA stating that their products were adulterated and mislabeled and should not be sold without authorization. The report stated that some companies even received import alerts ordered by the US FDA, authorizing US Customs and Border Protection agents to seize their products.
NJOY also noted that a study funded by Altria found that flavored e-cigarette products still account for more than 97% of the California market despite the Golden State’s ban.
NJOY also said that more manufacturers, distributors and retailers may still be named as defendants. The defendant companies could not immediately be reached for comment Thursday evening.
Altria Group announced plans to acquire NJOY for $3.25 billion in March. At the time, Altria said in a statement that the U.S. e-cigarette market was on the verge of a “multi-year transition period” as the FDA makes marketing decisions on a large number of pending product applications.
NJOY received FDA marketing authorization (known as a marketing authorization order, or MGO) for six of its products in 2022, including its leading brand NJOYACE. Late last year, the U.S. Supreme Court rejected a request by cigarette companies to block California’s ban. Tobacco companies say federal law prohibits the sale of flavored tobacco products. California believes flavored tobacco products play a key role in youth addiction because those who start smoking flavored tobacco are more likely to become long-term users, according to court documents. NJOY is represented by Arnold & Porter’s Lauren S. Wulfe, Kristina Iliopoulos, John C. Massaro, David E. Kouba and Paul W. Rodney. Attorney information for the defendants was not immediately available. The case is NJOY LLC v. i Miracle (HK) Limited et al., case number 2:23-cv-08798, which was heard in the U.S. District Court for the Federal District of California. ——Additional reporting by Al Barbarino and Emily Field. Edited by Caitlin Wolper.
3. Researchers identify e-cigarette flavors for smoking cessation
A recent survey showed that the most commonly used quit smoking flavors in the United States are fruit, baked goods and chocolate.
A team of European researchers affiliated with the Center of Excellence for Accelerated Harm Reduction (CoEHAR), the University of Western Attica and the University of Patras conducted an online survey of approximately 70,000 adult e-cigarette users in the United States. To compare flavor use between current smoking e-cigarette users (dual use) and former smoking e-cigarette users and specifically examine flavor use patterns among former smoking e-cigarette users while quitting smoking.
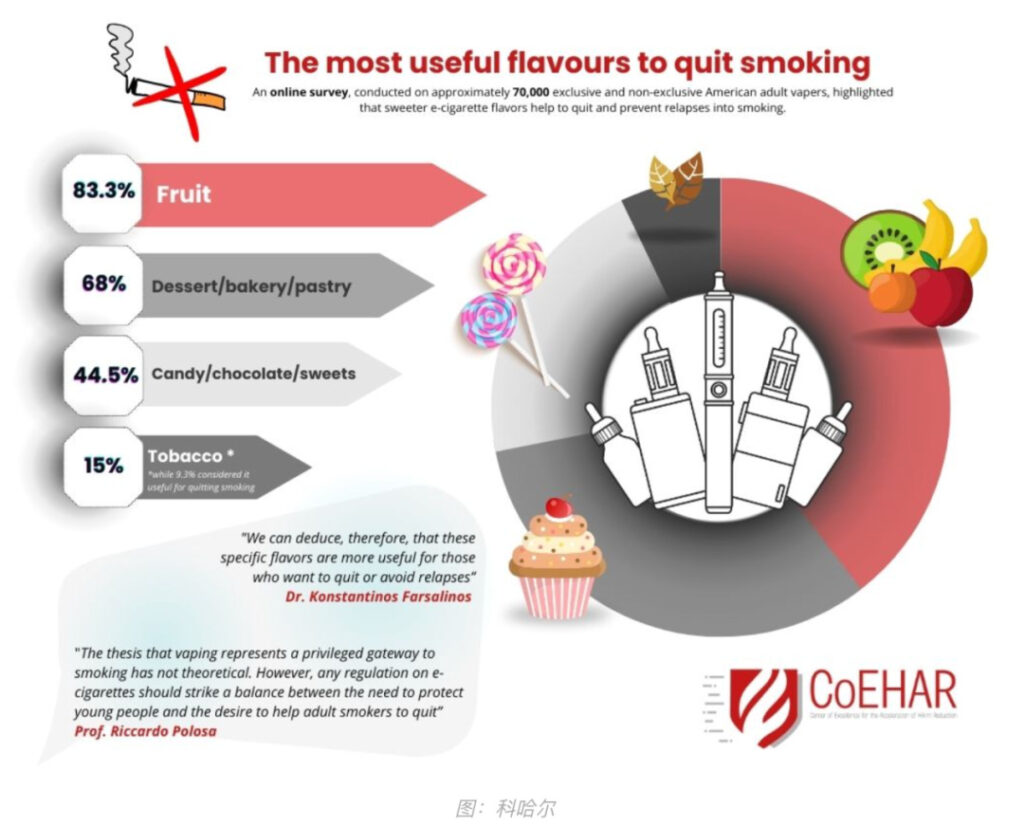
“In terms of sample size, this is the largest survey ever conducted on e-cigarette use,” study author Konstantinos Farsalinos said in a statement. “Most interesting. The data is that when smokers decide to quit smoking using risk-modifying electronic tools, they gravitate toward flavors different from tobacco, with a clear preference for fruit, dessert, and chocolate flavors. Therefore, we can infer that these specific flavors are more attractive to those who want to quit smoking. It is more useful for people who want to quit smoking or avoid relapsing.”
When it comes to regulating e-cigarette flavors, CoEHAR founder Riccardo Polosa urged lawmakers to balance the need to protect young people with the desire to help adult smokers quit.
4. RJR complaints could undermine the e-cigarette industry

The impact could be far-reaching. RJ Reynolds has filed a lawsuit with the U.S. International Trade Commission (ITC) alleging unfair importation by multiple manufacturers, distributors and retailers of a variety of popular disposable vaping devices. It’s one of several recent actions Reynolds has taken to remove rival vaping products from store shelves.
Reynolds asked the U.S. International Trade Commission to investigate and issue an exclusion order to prevent further imports of disposable e-cigarette products into the United States. Several legal scholars told Tobacco Reporter that if the ITC agreed with Reynolds, all flavored disposable e-cigarette devices without marketing authorization could be stopped at the border and blocked from entering the U.S. market.
Reynolds wants the U.S. International Trade Commission to issue a permanent “cease and desist order” prohibiting any company from selling illegal e-cigarette products. The move would drive nearly the entire e-cigarette industry underground, except for products owned by big tobacco companies like Reynolds, which have received marketing orders from the FDA.
Reynolds’ indictment specifically named several companies as “illegal disposable e-cigarette dealers,” including Breeze, ElfBar, EscoBar, Hyde, PuffBar, and R&M disposable e-cigarettes as “manufacturers, importers, distributors and Retailers”.
Also named were several well-known disposable e-cigarette wholesalers and retailers in the United States, including ElementVape, FlawlessVape, Magellan Technology, Mi-One Brands, PricePoint Distributors and VapeSourcing.
The ITC complaint accuses all unauthorized e-cigarette product manufacturers of importing “illegal disposable e-cigarettes” in violation of Section 337 of the Tariff Act of 1930. Specifically, Reynolds alleges that these businesses either falsely represented that their products were authorized for sale, failed to comply with federal law requiring registration and reporting requirements and sales restrictions imposed by the U.S. government, or violated customs laws and regulations.
Reynolds owns the Vuse e-cigarette brands, including VuseAlto. Last week, the FDA issued a denial of marketing order ordering Alto menthol refill packs to be withdrawn from the market. The Alto device and tobacco-flavored cartridges are still under review by the agency. Among the 23 products currently authorized by the FDA are two older Vuse e-cigarettes, the Solo and Vibe models (and their tobacco-flavored refills).
Reynolds also said it has the ability to fill any gaps in the market if illegal products are removed. “Reynolds has the ability to meet any increase in demand if the accused products are excluded from importation,” the complaint states. “Reynolds is willing to meet any increased demand and can do so within a commercially reasonable time because it has provided The industry offers a wide range of ENDS products as well as oral tobacco and nicotine products.”
The ITC has yet to make a decision on the complaint filed on October 13.
5. HTP: EU rulemaking challenged in court

The European Commission is set to face a legal challenge over its attempts to restrict the sale of heated tobacco products (HTPs).
On November 3, 2022, the European Union issued a directive banning the use of flavored HTP across the Union. The ban covers all flavors except tobacco and will officially take effect on November 23, 2022. EU member states have until July 23, 2023 to translate the rule into national legislation.
When Ireland did so, it was challenged in the Irish High Court by PJ Carroll & Co. and Nicoventures Trading. Nicotine companies argue that the European Commission exceeded its powers under tobacco product legislation approved by the European Council and European Parliament. They said the committee’s decision was based on political rather than legal grounds.
In his judgment, Irish High Court Judge Cian Ferriter noted that the Commission effectively banned “a new category of tobacco products on the market that did not exist when the Tobacco Products Directive was enacted in 2014 and that this was not a separate policy and health assessment.” Theme of……”.
“To say the least, this involves a political choice that is open only to the EU legislators and not to the European Commission,” Ferrit said.
According to Eureporter, a Dublin court has now transferred the case to the European Court of Justice in Luxembourg.
Nicotine companies and Ireland’s High Court are not the first to express concerns about excessive regulation. When the European Commission adopted the directive in 2022, four EU member states objected, arguing that it dealt with “fundamental elements reserved for European legislators.”
6. New proposals to combat illegal trade

House Ways and Means Committee Chairman Joey Salceda proposed new legislation to combat the illegal trade in cigarettes, the Philippine News Agency reported.
Among other things, these measures will address the leakage of tobacco smuggled, declared for export or transshipped through the country’s economic zones, as well as the manufacture of counterfeit cigarettes.
The illegal tobacco market in the Philippines has been booming recently. If the illegal tobacco trade continues on its current trajectory, the government expects to lose 60.6 billion Philippine pesos ($1.06 billion) in revenue this year.
Salceda noted that tax collections fell 7.8% to P160.4 billion in 2022, leaving the government P31.2 billion short of the P191.6 billion target for 2022.
Salceda said illegal cigarettes are “easily available” in every trade sector. “There are no challenges in buying these brands,” he said. “And they sell for as little as a fifth of the price of legal cigarettes. Legal people don’t stand a chance. Even fakes of premium brands are becoming increasingly available. On the same online shopping site, they are openly on sale for half price And allegedly fake products with the same flavor.
“At the same time, the revenue base will continue to shrink, and prevalence may actually increase due to cheaper illegal alternatives. This is a serious national crisis. For better or worse, our advocacy for higher taxes is increasing illegal The attractiveness aspect of the sector plays a role. We have a responsibility to help address that,” he said.
7. Mint Rules Advance U.S. Budget Office

According to CNN, the U.S. FDA’s Center for Tobacco Products (CTP) has sent the rule banning the sale of menthol cigarettes and flavored cigars to the White House Office of Management and Budget (OMB) for final review.
The final rule will be issued after the final regulatory steps.
The American Lung Association (ALA) said this rule may be the most important action taken by the FDA in the 14 years since it obtained tobacco regulatory authority.
“This is an important step toward banning these products,” said Erika Sward, ALA’s assistant vice president of national advocacy. “Indeed, it’s important.”
According to a study published in 2022 in Tobacco Control, banning menthol cigarettes would save up to 654,000 lives in the United States over 40 years, including 255,000 lives of members of the Black community.
The Campaign for Tobacco-Free Kids calls on the White House and OMB to expedite the review and issue a final rule by the end of 2023.
8. Philip Morris International (PMI): COP10 missed opportunities

Philip Morris International is concerned that participants at the upcoming meeting of the parties to the World Health Organization Framework Convention on Tobacco Control (FCTC) will pursue bans on non-combustible tobacco products, according to an article in The Guardian.
“Major portions of the agenda and meeting documents have been made public,” PMI senior vice president of external affairs Gregoire Verdeaux wrote in an email. “Unfortunately, they reconfirm all our fears that this meeting may still be tobacco-related. The greatest missed opportunity in the history of control… The WHO’s agenda is nothing more than a systematic, methodical, prohibitive attack on smoke-free products.”
Without “reasonable, constructive outcomes,” Vidor wrote, “the World Health Organization will irreversibly undermine public health’s historic opportunity to realize that smoke-free products, when properly regulated, can control tobacco products faster than tobacco control combined.” to accelerate the decline in smoking rates.”
While tobacco companies were not invited to the FCTC COP, Verdeaux said he would be in Panama to “publicly condemn the absurdity of being excluded, and today’s PMI is undoubtedly the most helpful WHO can have.” private partners.” Fighting Smoking. “
Last year, PMI generated $10.19 billion in revenue from products such as heated tobacco and e-cigarettes.
9. The CBD and nicotine e-cigarette markets diverge

According to an article published by Bloomberg, the cannabis vaping and tobacco vaping industries in the United States are following very different paths, largely influenced by regulatory dynamics. While tobacco e-cigarettes face increasing restrictions on nicotine content, flavored products and youth use, the cannabis e-cigarette industry benefits from limited regulation, making it a significant player in the cannabis market.
The U.S. FDA’s ban on flavored e-cigarette products has been particularly detrimental to the nicotine e-cigarette industry, affecting well-known brands such as VuseAlto, which was recently the subject of an FDA marketing denial order.
Marijuana vaping, by contrast, is growing rapidly, with sales estimated at $6.8 billion this year. These numbers likely understate the market due to the impact of illicit marijuana sales that are difficult to track. However, as marijuana legalization advances state by state, questions are beginning to arise about when the industry might face more regulation.
Some suppliers in the nicotine e-cigarette business see huge potential in the cannabis industry, as the U.S. e-cigarette parts market alone is an estimated $700 million opportunity. Meanwhile, the rise of disposable e-cigarettes also poses challenges, as concerns about waste and youth appeal lead to potential regulatory hurdles.
10. New tax fairness bill will make e-cigarettes more expensive than smoking
A new bill introduced in both houses of Congress would impose a first-ever federal tax on vaping products and nicotine pouches. The bill’s authors say its purpose is to harmonize tax rates on all consumer nicotine products based on nicotine content, but in practice this would make e-cigarettes more expensive than smoking cigarettes.
Thirty-three states and federal territories currently impose some kind of e-cigarette tax, many of which tax nicotine pouches. But there are currently no federal taxes on tobacco-free nicotine products such as e-cigarettes.
If the Tobacco Tax Fairness Act passes, it would impose a federal tax of $100.66 per 1,810 milligrams of nicotine on all tobacco-free nicotine products. Nicotine is about 5.5 cents per milligram. Consumers vaping 50 mg/ml nicotine salt e-liquid will pay an additional $2.75 per ml of juice, or $82.50 for a 30 ml bottle, in addition to the regular product price and any state or local taxes.
The price of a 60ml bottle of 12mg/ml e-liquid would increase by about $40, while the bill would increase the price of a 5ml disposable e-cigarette by about $14. A single JUUL pod is taxed at a higher rate than a pack of cigarettes ($2.25 vs. $2.00), while a pack of 20 nicotine pouches will cost an additional $2.00. For a DIY e-cigarette machine, the tax for a one-liter bottle of 100 mg/ml nicotine will be more than $5,000!
The Consumer Advocates for Smoke-Free Alternatives (CASAA) has issued a nationwide call to action, encouraging e-cigarette users and other nicotine consumers to contact their members of Congress to voice their opposition to the shocking new tax proposal.
For DIY e-liquid manufacturers, a one-liter bottle of 100 mg/ml nicotine would be taxed at more than $5,000.
The Senate bill is S2929, introduced by Illinois Democrat Dick Durbin, a longtime enemy of e-cigarettes, and co-sponsored by eight other Senate Democrats. The House version, HR5715, was introduced by Illinois Rep. Raja Krishnamoorthi, also a Democrat who has been involved in many anti-vaping legislative efforts. Both bills were introduced on September 26.
A few days ago, Durbin introduced the Caring Moms Act, which also includes new e-cigarette and tobacco tax proposals. However, Durbin did not mention taxes in his press statement about the CARES Act.
Durbin has introduced a number of similar “tax fairness” bills in previous congressional sessions. Language from his Tobacco Tax Fairness Act of 2021 was included in Democrats’ year-end spending bill two years ago until Sen. Catherine Cortez Masto, D-Nev., made it clear within days Delete it.
The Tobacco Tax Fairness Act of 2023 would also double federal taxes on cigarettes, roll-your-own cigarettes, and cigarillos, and increase taxes on moist snus and pipe tobacco by up to 2,000 percent. Moist snuff is clearly safer than any form of burning tobacco.
In a press release touting the new bill, the authors boasted that it was endorsed by the Campaign for Tobacco-Free Kids, the American Lung Association, the American Heart Association, the American Public Health Association, the American Academy of Pediatrics, Trust for America’s Health, and counties and cities nationwide Association of Health Officials.

